Project Title:
Simultaneous, Proportional and Independent sEMG-Based Hand-Wrist Prosthesis Control
Summary:
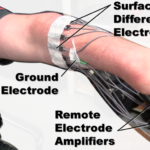
Surface electromyogram (sEMG) controlled powered hand/wrist prostheses are used by upper-limb amputees to return partial upper-limb function. For patients without a hand and wrist, conventional prostheses can use surface EMG amplitudes from the residual forearm to control hand closing and opening. Additional degrees of freedom (DoFs), like wrist rotation, are not controlled at the same time in these systems. Rather, prostheses apply EMG-based or mechanical mode switching, so that the same EMG sites sequentially control the added functions. This can be a problem, as many basic tasks, like drinking from a cup or combing hair, require the control of two or more joints at once. It was reported that the ability to control “… coordinated motions of two joints at the same time” was the second highest priority improvement desired by patients. The first priority was for a rotating wrist—a necessary prerequisite for two DoF control, and a device that is already commercially available.
This project aims to provide more intuitive control to support upper-limb prostheses with multiple degrees of freedom. In our Phase 1 work, we demonstrated the feasibility of a system for electrode site selection that controls two DoFs with only four commercial electrodes. A calibration procedure using a 16-channel EMG system without the prosthetic socket is used in the laboratory (or implementation) to select EMG sites and relate their electrical activity to 2 DoFs. In this Phase 2 application, we propose to transition this method to a commercial product in the prosthetist’s office. Aim 1 will develop the necessary hardware/software to prototype the embedded commercial controller as well as the clinical fitting/training system. Aim 2 will refine the site selection and control algorithms. Our Phase 2 work will focus on utilizing only the affected limb (for a more direct control relationship) and on determining ways to rapidly calibrate a 2-DoF controller (in the laboratory and in the field). Aim 3 will evaluate the prototype devices and algorithms developed in Aim 1 and 2 in a laboratory study, then in a pilot field study, facilitating commercialization.
Publications:
Funding:
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R43HD076519 and R42HD076519. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Completed